Metal coupling on rusting of iron chemistry project pdf
Metal coupling on rusting of iron chemistry project pdf
Introduction. Corrosion is what happens to metals when they are exposed to water and oxygen in the environment. When iron or steel corrodes, the iron forms reddish brown colored oxides and hyrdoxides: what we commonly refer to as “rust.”
Chemistry of Rusting Iron. Rust is a general term for iron oxides formed by the reaction of iron with oxygen. Several forms of rust are distinguishable visually, and form under different circumstances.
metals. The purpose of my science project was to use simple, inexpensive and non-hazardous household The purpose of my science project was to use simple, inexpensive and non-hazardous household materials to study the relationship between acid and rust formation.
Corrosion is the process in which a metal is destructed as a result of its reaction with environment. Corrosion of iron is known as rusting. Rusting is the hydrated ferric oxide.
1 Corrosion and Corrosion Protection Peter Maa ß • Contact corrosion (aka dissimilar metal corrosion) Occurs at contact surfaces of different metals; the acceleratedly corroding metal area is the anode of the corrosion element. • Intergranular corrosion Corrosion in or adjacent to the grain boundaries of a metal. The standard mentioned above describes altogether 37 types of corrosion
THE RUSTING OF IRON Introduction There are ninety two elements found in nature. More than 80% of these are known as metals or metallic elements. Metal atoms loose some of… More than 80% of these are known as metals or metallic elements.
Rust occurs when iron or its alloys, such as steel, corrode. The surface of a piece of iron will begin to corrode first in the presence of oxygen and water. Given enough time, any piece of iron will change entirely into rust and disintegrate.
Corrosion occurs through the oxidation of metals in our environment. Most metals corrode, some more rapidly than others, depending on that environment. Students are familiar with rust, the iron oxide corrosion product of steel. The form and amount of corrosion that occurs depends on factors such as: • Amount of water in the environment • Presence of salt or other chemicals • State of
chemistry. All engineering metals react with their environment, and some are more reactive than others. The rate of reaction depends on the environment; when dis-similar metals are electrically connected to each other, each interacts with the environment, and the more reactive metal takes the brunt of the reaction as shown in the iron/zinc couple in Figure 1. While corrosion in a couple always
To Study the Effect of Metal Coupling on the Rate of Corrosions Chemistry Science Fair Project Experiments , Chemistry Models, Exhibition Ideas, Expo Topics for Kids and also Organics Chemistry Science ideas for CBSE, ICSE, Middleschool, Elementary School for 5th, 6th, 7th, 8th, 9th and High School Students.
CHEMISTRY PROJECT by Abinav Muthukrishnan on Prezi
chemistry project on effect of metal coupling on rusting
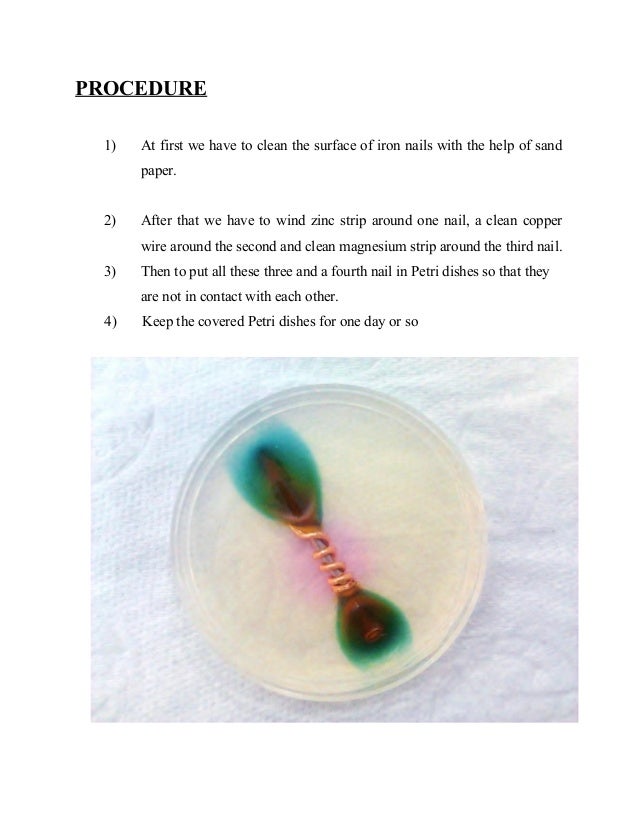
3. Then to fill the Petri dishes with hot agar agar(a jelly) solution in such a way that only lower half of the nails are covered with the liquids. Cover the Petri dishes for one day or so. 4. The liquid set to a gel on cooling. Two types of patches are observed around the rusted
Chemistry Corrosion Iron Iron is the fourth most common element in soil, comprising 5% of the earth’s crust. The iron in soil is usually found in the soluble cation form (Fe 2+ ). This r educed form is more common because of the lower levels of oxygen in soil. This ion can be readily absorbed by plants. W hen high levels of oxygen are present in the air surrounding soil particles, oxidation
corrosion of metals is an electrochemical process, it is also not surprising that the Corrosion Division is one of the oldest divisions within ECS. The Division was established in 1942, but corrosion has been an important topic in the Society since 1903. Reviews of the early literature and history of the Division were prepared by Uhlig for the 50 th and 75 anniversaries of the Society2,3 and a
Rusting is the process in which iron turns into iron oxide. It happens when iron comes into contact with water and oxygen. The process is a type of corrosion that occurs easily under natural… It happens when iron comes into contact with water and oxygen.
I. N V E S T I A T O R Y P R O J E C T AIM— study of effect of metal coupling on rustng of iron I N V E S T I A T O R Y P R O J E C
Thus corrosion can proceed by the coupling of reactions (1) and (6). In electrochemical terminology, an electrode at which an oxidation reaction occurs is called an anode. The process of oxidation involves a loss of electrons by the reacting species, as occurs in the metal dissolution reaction (1). Thus an area of a corroding metal where metal dissolution occurs is an anode and metal
Rusting of Iron Project Report . Topics: Oxygen Metals and alloys undergo rusting and corrosion. The process by which some metals when exposed to atmospheric condition i.e., moist air, carbon dioxide form undesirable compounds on the surface is known as corrosion. The compounds formed are usually oxides. Rusting is also a type of corrosion but the term is restricted to iron or products
Iron-Aluminium Iron-Zinc Iron-Cooper Does not rusts Does not rusts Nail rusts Iron-Magnessium Does not rusts Result and Inference The – experiment performed on coupling has shown that rusts can be avoided by coupling of iron with more electropositive metal. can be saved from rusting . so it is oxidised leaving iron nail safe. In this experiment zinc was more electropositive. In this way
You use iron couplings and fittings with iron pipe. Placing a block is a little trickier, but is a requirement. Blocks between structural members can be dense plastics or hard rubber. Weber State University makes it a performance requirement to place non-conductive blocks between exterior iron railings and dissimilar metals to stop galvanic action.

Metal coupling affects the rusting of iron . If the nail is coupled with a more electro-positive metal like zinc, magnesium or aluminium rusting is prevented but if on the other hand , it is coupled with less electro – positive metals like copper , the rusting is facilitated.
Chemistry investigatory project on effect of metal coupling on rusting of iron Ask for details ; Follow Report by Bimalpahari4571 22.03.2018
When coupling of these metals was done each couple showed some difference in their corrosion with respect to each metal kept alone. Iron + Aluminium couple has the highest rate of corrosion while iron +Zinc couple has the lowest rate of corrosion.

corrosion resistance of the cast iron increases dramatically (Rana et. al., 2001). Carbon in cast iron can exist in two forms, namely as free graphite or combined with some of the iron to form iron …
There are several projects that can be done to find out the effectof coupling on rust. One example would be to experiment on severaldifferent types of alloys to see which rusts least.
iC B S E . c o m IADEX 1. CertiIicate 2. Acknowledgement 3. Objective Aim : Project on effect of metal coupling on rusting of iron
That leads to the formation of rust For iron to form rust, oxygen and water must be present. You can test your knowledge with the experiment that addresses one or more of these substances.
effect of metal coupling on rusting of iron?? Yahoo Answers
Corrosion of metals Introduction To ensure long and troublefree operation in a media it is of utmost importance to have knowledge about corrosion and the effect it can have on the product and the system in the operating environment.
The atmospheric rusting of iron and steels can be considered as wet corrosion in the thin water film formed on the surface of iron and steel and its physical and electrochemical mechanisms are …
The cover photo depicts galvanically induced corrosion of 316 stainless steel caused by the deposition of active carbon used as a decolorizing agent for the sugar solution in contact with the metal.
Iron-oxidizing bacteria are chemotrophic bacteria that derive the energy they need to live and multiply by oxidizing dissolved ferrous iron. They are known to grow and proliferate in waters containing iron concentrations as low as 0.1 mg/L.
24/01/2008 · Best Answer: The use of a more reactive metal to prevent oxidation of Iron depends upon the relative electromotive force of the oxidation reactions. Extremely electropositive metals (like Lithium, Sodium and Potassium) are not used because they will even react with water (often violently). Metals …
In this project the aim is to investigate effect of the metals coupling on the rusting of iron. Metal coupling affects the rusting of iron . If the nail is coupled with a more electro-positive metal like zinc, magnesium or aluminium rusting is prevented but if on the other hand , it is coupled with less electro – positive metals like copper , the rusting is facilitated.
This is an experiment from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. Preventing rusting Experiment Preventing rusting. Class practical or demonstration. In this experiment students protect iron nails using a variety of methods including painting, greasing and sacrificial protection. The nails are placed in test-tubes and covered
Rusting is the corrosion of iron and steel, as that is exactly what the rust is doing, corroding or destroying the metal through a chemical reaction. Essentially, if water or moisture comes into contact with iron or an alloy of iron, such as steel, which hasn’t been protected by any form of coating such as paint, a chemical reaction takes place as the water causes the metal to react with – example of metal heat treatment Projects are available in PDF format inside archive. Chemistry Project on Metal coupling in rusting of Iron Chemistry Project on Electrolysis of Potassium Iodide (KI) Chemistry Project to Compare Rate of Fermentation Chemistry Project on Fatty Material of Different Soap Samples Chemistry Project on Extraction of Essential Oil from Aniseed Chemistry Project on Analysis of Cold Drinks
After twelve hours, steel wool in water, vinegar and bleach has more rust than the control, because the vinegar strips of wool, sheeting and bleach accelerates the reaction between dissolved oxygen and iron with the formation of the oxide.
AIM OF THIS PROJECT In this project the aim is to investigate effect of the metals coupling on the rusting of iron. Metal coupling affects the rusting of iron. If the nail is coupled with a more electro-positive metal like zinc, magnesium or aluminium rusting is prevented but if on the other hand, it is coupled with less electro –positive metals like copper, the rusting is facilitated.
Standard expressions for corrosion rate In most cases, aside from contamination problems, the primary concern where corrosion is present, is the life (usually in years) of metals in question.
Rust is soft and crumbly and readily flakes off exposing more metal to water and oxygen (in air) i.e. the rusting chemistry just keeps on eating the metal away, eventually, completely! Corroded components or structures weakened, adding further costs in rust treatment or replacement.
Corrosion is the deterioration or destruction of metals and alloys in the presence of an environment by chemical or electrochemical means. In simple terminology, corrosion processes involve reaction of metals …
When coupling of these metals was done each couple showed some difference in their corrosion with respect to each metal kept alone. Iron + Aluminium couple has the highest rate of corrosion while iron +Zinc couple has the lowest rate of corrosion. Rate of corrosion of each couple is in the order of Iron + Aluminium > Brass + Zinc> Iron + Zinc
Corrosion (e.g. rusting of iron) take place rapidly at bends, scratches, nicks and cuts in the metal. (v) Presence of electrolytes. Electrolytes, if presents, also increase the rate of corrosion.
Chemistry Project on Metal coupling in rusting of Iron. Chemistry Projects.Most corrosion resistant metal project. Hydroxides: what we commonly name it rust.
Rust is a type of corrosion. It happens to iron and its alloys when it is exposed to air or water for a long time. Rust slowly decomposes iron into other chemicals, because of a …
Name(s) Project Number Kamran M. Jamil J0510
Rust Simple English Wikipedia the free encyclopedia

Electrochemical Theory of Rusting Mastering Chemistry Help
10 the Rusting of Iron [DOC Document]

(322737428) 123307105 Effect of Metal Couon Rusting of Iron
The Effects of Metal Couplings on the Rusting of Iron


To Study the Effect of Metal Coupling on the Rate of
Chemistry investigatory project on effect of metal
– Chemistry Project to Study Effect of Metal Coupling on
Effect of Metal Coupling on the Rusting of Iron

To Study the Effect of Metal Coupling on the Rate of
chemistry project on effect of metal coupling on rusting
metals. The purpose of my science project was to use simple, inexpensive and non-hazardous household The purpose of my science project was to use simple, inexpensive and non-hazardous household materials to study the relationship between acid and rust formation.
That leads to the formation of rust For iron to form rust, oxygen and water must be present. You can test your knowledge with the experiment that addresses one or more of these substances.
Corrosion is the deterioration or destruction of metals and alloys in the presence of an environment by chemical or electrochemical means. In simple terminology, corrosion processes involve reaction of metals …
3. Then to fill the Petri dishes with hot agar agar(a jelly) solution in such a way that only lower half of the nails are covered with the liquids. Cover the Petri dishes for one day or so. 4. The liquid set to a gel on cooling. Two types of patches are observed around the rusted
You use iron couplings and fittings with iron pipe. Placing a block is a little trickier, but is a requirement. Blocks between structural members can be dense plastics or hard rubber. Weber State University makes it a performance requirement to place non-conductive blocks between exterior iron railings and dissimilar metals to stop galvanic action.
Rusting of Iron Project Report . Topics: Oxygen Metals and alloys undergo rusting and corrosion. The process by which some metals when exposed to atmospheric condition i.e., moist air, carbon dioxide form undesirable compounds on the surface is known as corrosion. The compounds formed are usually oxides. Rusting is also a type of corrosion but the term is restricted to iron or products
The atmospheric rusting of iron and steels can be considered as wet corrosion in the thin water film formed on the surface of iron and steel and its physical and electrochemical mechanisms are …
24/01/2008 · Best Answer: The use of a more reactive metal to prevent oxidation of Iron depends upon the relative electromotive force of the oxidation reactions. Extremely electropositive metals (like Lithium, Sodium and Potassium) are not used because they will even react with water (often violently). Metals …
Iron-Aluminium Iron-Zinc Iron-Cooper Does not rusts Does not rusts Nail rusts Iron-Magnessium Does not rusts Result and Inference The – experiment performed on coupling has shown that rusts can be avoided by coupling of iron with more electropositive metal. can be saved from rusting . so it is oxidised leaving iron nail safe. In this experiment zinc was more electropositive. In this way
AIM OF THIS PROJECT In this project the aim is to investigate effect of the metals coupling on the rusting of iron. Metal coupling affects the rusting of iron. If the nail is coupled with a more electro-positive metal like zinc, magnesium or aluminium rusting is prevented but if on the other hand, it is coupled with less electro –positive metals like copper, the rusting is facilitated.
When coupling of these metals was done each couple showed some difference in their corrosion with respect to each metal kept alone. Iron Aluminium couple has the highest rate of corrosion while iron Zinc couple has the lowest rate of corrosion. Rate of corrosion of each couple is in the order of Iron Aluminium > Brass Zinc> Iron Zinc
THE RUSTING OF IRON Introduction There are ninety two elements found in nature. More than 80% of these are known as metals or metallic elements. Metal atoms loose some of… More than 80% of these are known as metals or metallic elements.
iC B S E . c o m IADEX 1. CertiIicate 2. Acknowledgement 3. Objective Aim : Project on effect of metal coupling on rusting of iron
chemistry. All engineering metals react with their environment, and some are more reactive than others. The rate of reaction depends on the environment; when dis-similar metals are electrically connected to each other, each interacts with the environment, and the more reactive metal takes the brunt of the reaction as shown in the iron/zinc couple in Figure 1. While corrosion in a couple always
Thus corrosion can proceed by the coupling of reactions (1) and (6). In electrochemical terminology, an electrode at which an oxidation reaction occurs is called an anode. The process of oxidation involves a loss of electrons by the reacting species, as occurs in the metal dissolution reaction (1). Thus an area of a corroding metal where metal dissolution occurs is an anode and metal
