Application of boyle’s law in industries
Application of boyle’s law in industries
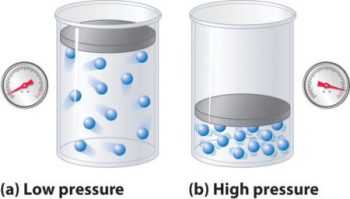
The Boyles Law At constant temperature, the pressure is inversely proportional to the volume of a definite amount of gas. This is known as the Boyles law. Robert Boyles (1627-1691), experimented with gas at constant temperature.
at a constant temperature, the volume of a gas remains the same no matter what the pressure exerted by that gas is. 3. An application of Boyle’s Law is… A) at a constant temperature, as the pressure on a gas increases, the volume decreases.
2015-02-18 · Charles’ Law Examples in Everyday Life. Charles’ law states that, It combines with Boyle’s law to form the ideal gas law,
applications of gas law The mechanics of a bicycle pump isgoverned by Boyle’s Law. A practical application illustrating Boyles Law would bethe action of a
2009-11-10 · How can i use Boyles law, and turn it into an industrial process? Boyle’s law is about Compressed air is widely used in many industrial applications:
Gas Laws with Examples 1. Boyle’s Law: other picture of gas laws applications of Amontons law of pressure daltons law of pressure example Amontons Law graph
2008-05-11 · Application of Archimedes’ Principle can u plz list down da application for Cartesian Diver n Plimsol line in a ship with the help Law of Reflection;
Looking for the Answer That what exactly Boyle Law is? Along with Boyle’s Law Equation – Examples – Formula & Definition, Explanation. Stay With us.
What Is Boyle’s Law? Boyle’s law describes the relationship between volume and pressure in a fixed mass of gas at a constant temperature. It states that the
2008-04-28 · Applications of Pascal’s Principle in Everyday Life Application of Pascal’s principle Boyle’s Law; Pressure Law;
Boyle’s Law is useful in scuba diving because it helps scuba divers predict how air will expand and compress with water pressure. Boyle’s Law and Scuba Diving. Search
Charles’ Law is an experimental gas law that describes how gases tend to expand when heated. The law states that if a quantity of gas is held at a constant pressure, there is a direct relationship between its volume and the temperature, as measured in degrees Kelvin. Think of it this way.
Classroom Diver Clinic
Themodynamics Boyle’s Law Bright Hub Engineering
Boyle’s Law. Boyle’s Law for gasses states: It is the principle that, for relatively low pressures, the absolute pressure of an ideal gas kept at constant temperature varies inversely with the …
CliffsNotes study guides are written by real teachers and professors, so no matter what you’re studying, CliffsNotes can ease your homework headaches and help you
Classroom NHS As a diver, Boyles law affects you every time you enter the water. Some applications of Boyle’s Law in action:
The most comprehensive technical information regarding the hose industry – Boyle’s Law. Technical References – Technical Literature – JGB Technical Information Center
Boyle’s Law, Dalton’s Law and Avogadro’s Law all have real-life implications in how your breathe You witness real life applications of at least one of these
The first one is Boyle’s Law, Charles’ Law is named after Jacques Charles but they are demonstrating an application of Charles’ Law.

BOYLE’S AND CHARLES’S LAWS. Boyle’s law holds that in isothermal conditions (that is, a situation in which temperature is kept constant), an inverse relationship exists between the volume and pressure of a …
2013-06-01 · i’v tried the Haber process but couldn’t d this because it uses Le Chatlier’s Principle as i need to talk about Boyle law ,pressure law or Charles law in my report
This is “The Ideal Gas Law and Some Applications”, section 6.5 from the book Beginning Chemistry (v. 1.0). For details on it (including licensing), click here.
Start studying Gas Laws. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Another real-life application of Boyle’s Law.
Boyle’s Law. Boyle’s Law (sometimes referred to as the Boyle-Mariotte Law) states that the absolute pressure and volume of a given mass of confined gas are inversely proportional, provided the temperature remains unchanged within a closed system. This can be stated mathematically as follows: [latex]P_1V_1=P_2V_2[/latex]
2009-06-03 · What are some everyday examples of Boyles Law? 1 following . 2 answers 2. Report Abuse. Use an everyday example to describe Boyle’s Law.
This has become a basic principle in chemistry now called Boyle’s law and is included as a special case into the more general ideal gas law Robert Boyle (1627-1691). In 1662, Robert Boyle discovered that when held at a constant temperature, the volume and pressure of a gas are inversely proportionate.
Boyle’s Law was founded by Robert Boyle (1627-1691), who was one of the great intellectuals of the century. Boyle’s Law in a nutshell states that for a gas, when the
gas industry should understand the Basic Gas Laws. (1) to the application of Basic Gas Laws. Boyle’s Law isa way to measure the change in volume that occurs

GAS LAWS Charles’s Law. In Boyle’s Law In this law the temperature stays constant. Pressure amd volume are inversely related; meaning that if pressure goes up
Examples of Boyle’s Law in Various Fields. Application of Boyle’s Law in Industries. Storage of Gases: Many industries store gases under high pressure.
Bhawani Industry offering Boyles Law Burette in Bengali Mohalla, Ambala, Haryana. Get contact details, address, map on Indiamart.
Boyle’s gas law states the volume of a gas is inversely proportional to the pressure, at a constant temperature. Here’s an example problem.
Underwater salvage operations rely on Boyles Law to calculate life application of Gay Lussac’s Law: In industries the detection of methane gas is done
Well, Boyle’s law basically tells us how pressure works with gases… It says the the pressure of a gas increases as the volume of the container decreases. So I'
Boyle’s Law Technical Reference – Technical Literature
Boyle’s Law Boyle’s Law experimental apparatus Teaching Supplies: (Application) Demonstrates Boyle’s law to study relationship of volume to Industries. Education;
Let’s review. The combined gas law is the combination of Boyle’s law, Practical Application: Components of a Telecommunications System Infographic;
Lyophilization Technology-Application of Scientific Principles. and biotechnology industries who develop and/or produce lyophilized (freeze-dried) Boyles Law
2015-01-14 · Chemistry: Boyle’s Law (Gas Laws) with 2 examples For a gas, pressure and volume are inversely proportional. If you keep everything else constant, then as
Such properties of gases are vital to society and industries for essential science based theory. Boyle’s Law sometimes referred as the Boyle-Mariotte Law is one of
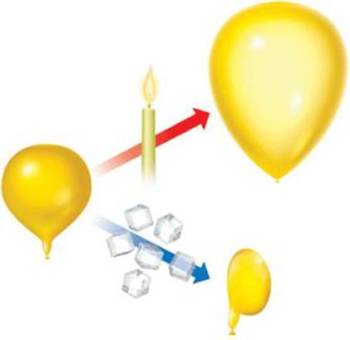
Quick Answer. Boyle’s law, the principle that the pressure on a gas is inversely proportional to its volume at constant temperatures, is demonstrable with everything from balloons to soda cans to SCUBA gear. Aerosol cans and syringes both rely on Boyle’s law in order to perform their functions as well. – what is fabrication engineering pdf Boyle’s Law describes the relationship between the pressure and volume of a system. As these two variables are indirectly proportional (as one goes up, one goes down), many applications utilize either the expansion or the compression of a gas when the pressure is either added or released.
Can someone explain to me Boyle’s Law using a real life situation? (Example: being in space or a scuba enough for a child to understand the concept of Boyle’s Law.
Definition and explanation of the gas laws: Boyle’s Law, Boyle’s Law: this article describes and defines Boyle’s Law with examples of using Boyle’s Ideal Gas Law to
Applications of the Ideal Gas Laws. By combining the gas laws of Boyle, Charles, Gay-Lussac, Use the Ideal Gas Law PV = nRT.
Some real-life applications of the law are: What is a real life application that demonstrates Gay-Lussac’s gas law? How does Boyle’s law relate to breathing?
Use/Application through History. Boyle’s Law can be explained with Daniel Bernoulli’s law (1738) and Rudolf Clausius’ kinetic-molecular theory (1857),
Boyle’s Law * describes the relationship between pressure and volume at constant temperature for a fixed mass * (number of molecules) of a gas. To understand Boyle’s law, it helps to visualize the behavior of gas particles (or molecules) in an enclosed space.
Boyle’s Law states that: Pressure is inversely propotional to Volume. But if we take an example of a balloon. As we fill air in it it’s pressure increases but it’s
2011-05-26 · Significance of Boyle’s Law, Importance of Boyle’s Law
INTRODUCTION Northeast Gas
Combined Gas Law Definition Formula & Example Video

Boyle’s Law Equation Examples – Formula & Definition
Chemistry Boyle’s Law (Gas Laws) with 2 examples

Boyle’s Law Boyle’s Law experimental apparatusTeaching
How can i use Boyles law and turn it into an industrial
Example of industrial process Physics Forums
https://en.wikipedia.org/wiki/Charles%27s_law
Boyle’s Law Experiment. Essay 496 Words
metal matrix composites in automotive applications – What Is Boyle’s Law? Reference.com
Gas Laws with Examples Online Chemistry Tutorials
What are some everyday examples of Boyles Law? Yahoo Answers
What is the importance of boyles law? Quora
Gas Laws Flashcards Quizlet
Real life application for combined gas law?
The most comprehensive technical information regarding the hose industry – Boyle’s Law. Technical References – Technical Literature – JGB Technical Information Center
Well, Boyle’s law basically tells us how pressure works with gases… It says the the pressure of a gas increases as the volume of the container decreases. So I'
Boyle’s Law Boyle’s Law experimental apparatus Teaching Supplies: (Application) Demonstrates Boyle’s law to study relationship of volume to Industries. Education;
applications of gas law The mechanics of a bicycle pump isgoverned by Boyle’s Law. A practical application illustrating Boyles Law would bethe action of a
The first one is Boyle’s Law, Charles’ Law is named after Jacques Charles but they are demonstrating an application of Charles’ Law.
Definition and explanation of the gas laws: Boyle’s Law, Boyle’s Law: this article describes and defines Boyle’s Law with examples of using Boyle’s Ideal Gas Law to
Can someone explain to me Boyle’s Law using a real life situation? (Example: being in space or a scuba enough for a child to understand the concept of Boyle’s Law.
Boyle’s Law was founded by Robert Boyle (1627-1691), who was one of the great intellectuals of the century. Boyle’s Law in a nutshell states that for a gas, when the

2008-04-28 · Applications of Pascal’s Principle in Everyday Life Application of Pascal’s principle Boyle’s Law; Pressure Law;
Boyles Law Burette View Specifications & Details of